
cGMP Cell Culture Supplement for Safe, Consistent, and Efficient Human Cell-Based Therapies
In 2010, after several years of success processing human platelet lysate (hPL) in-house at the Human Cell Therapy Laboratory (HCTL) at the Mayo Clinic, Dr. Allan Dietz licensed the IP for hPL and for a glioblastoma (GBM) cancer treatment and founded Mill Creek Life Sciences (MCLS). The development of our human platelet lysate arose from the need to find a suitable cGMP alternative to fetal bovine serum.
Human platelet lysate was not just a substitute, but a far superior alternative to Fetal Bovine Serum (FBS) and most serum free commercial solutions. Currently, hPL production is Mill Creek’s main operation. Our products have been used in Mesenchymal Stromal Cells (MSC) and other cellular therapy clinical trials globally in over 1,000 patients.
Mill Creek's Human Platelet Lysate
Our hPLs are made from expired human platelets, collected at AABB & FDA certified U.S. blood banks, that were intended for use in human transfusion. The donor units are transported frozen to MCLS and then pooled in large batch sizes and manufactured according to our proprietary methods to produce a consistent product.
Our commercially available human platelet lysate is a consistent and reliable alternative to FBS, Human AB Serum, defined medias, and lab-based platelet lysates. With the increasing interest in human cellular therapy, our hPLs meet the need for cell culture supplements that are animal serum-free, xeno-free, and cost effective.
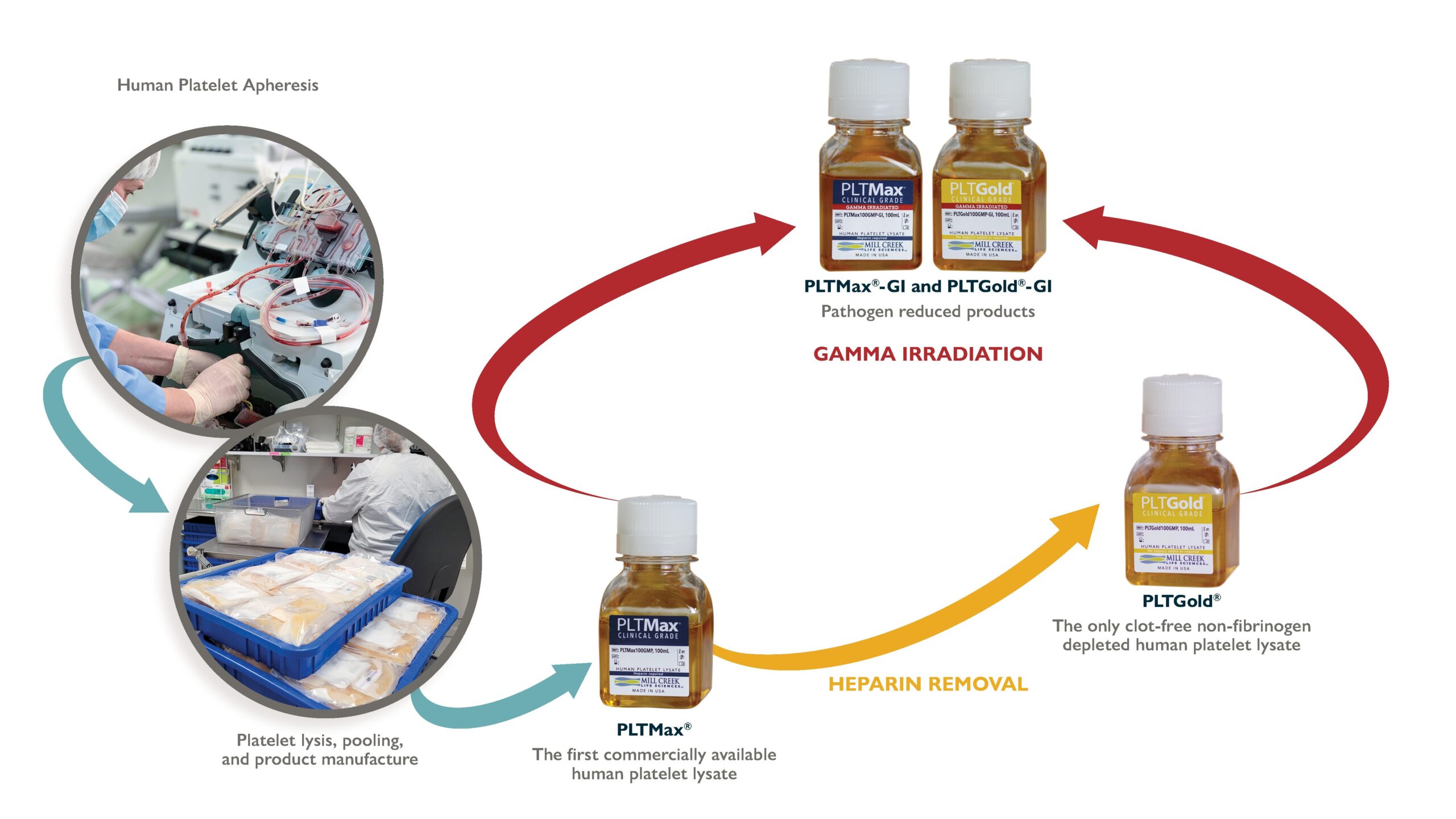
PLTMax®
Our initial product, derived from expired human platelets to provide an entirely human-derived, rich growth media supplement.
Gamma Irradiated Products Available for Added Safety
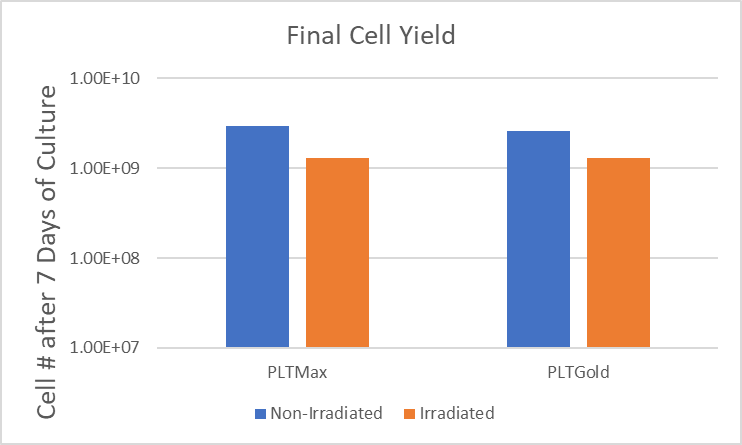
With the emergence of new infectious diseases, transmission of potential pathogens via blood transfusion or the use of blood derived products in the clinic can be a concern. Development of human platelet lysates with a pathogen reduction process helps to improve the safety profile for these cell-based therapies. Mill Creek Life Sciences developed PLTMax®-GI and PLTGold®-GI, human platelet lysates that have been treated with gamma irradiation for pathogen reduction in a process that maintains product quality and efficiency. MCLS validated the gamma process with a 5-virus viral clearance study that demonstrated viral inactivation with each of the viruses.

Benefits of MCLS Human Platelet Lysate
Initially developed for culturing MSCs, the rich nature of our hPLs allowed us to expand the applications outside of the world of regenerative medicine. Currently, several laboratories and hospitals around the world are using our products to grow many types of cells and in many different applications, including CAR-T based cancer immunotherapy.


