Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive form of malignant brain tumor. Standard treatment for GBM is surgical resection, followed by radiation therapy and chemotherapy with Temozolomide (TMZ). However, 90% of patients still experience tumor recurrence with a median overall survival of only 14.6 months.
The Mayo Clinic in Rochester, MN, USA, developed an autologous Dendritic Cell (DC) vaccine, which was evaluated in two phase I clinical trials for newly diagnosed and recurrent GBM patients. The vaccine technology is comprised of two components: Allogeneic tumor lysates with classic GBM antigens and autologous dendritic cells capable of inducing an immune response.
Mill Creek Life Sciences (MCLS) obtained the license to this technology, which we aim to optimize and adapt for manufacturing of the DC vaccine therapy on a wider scale, allowing us to produce safe and effective vaccines for larger clinical trials and eventual commercialization.
Technology Overview
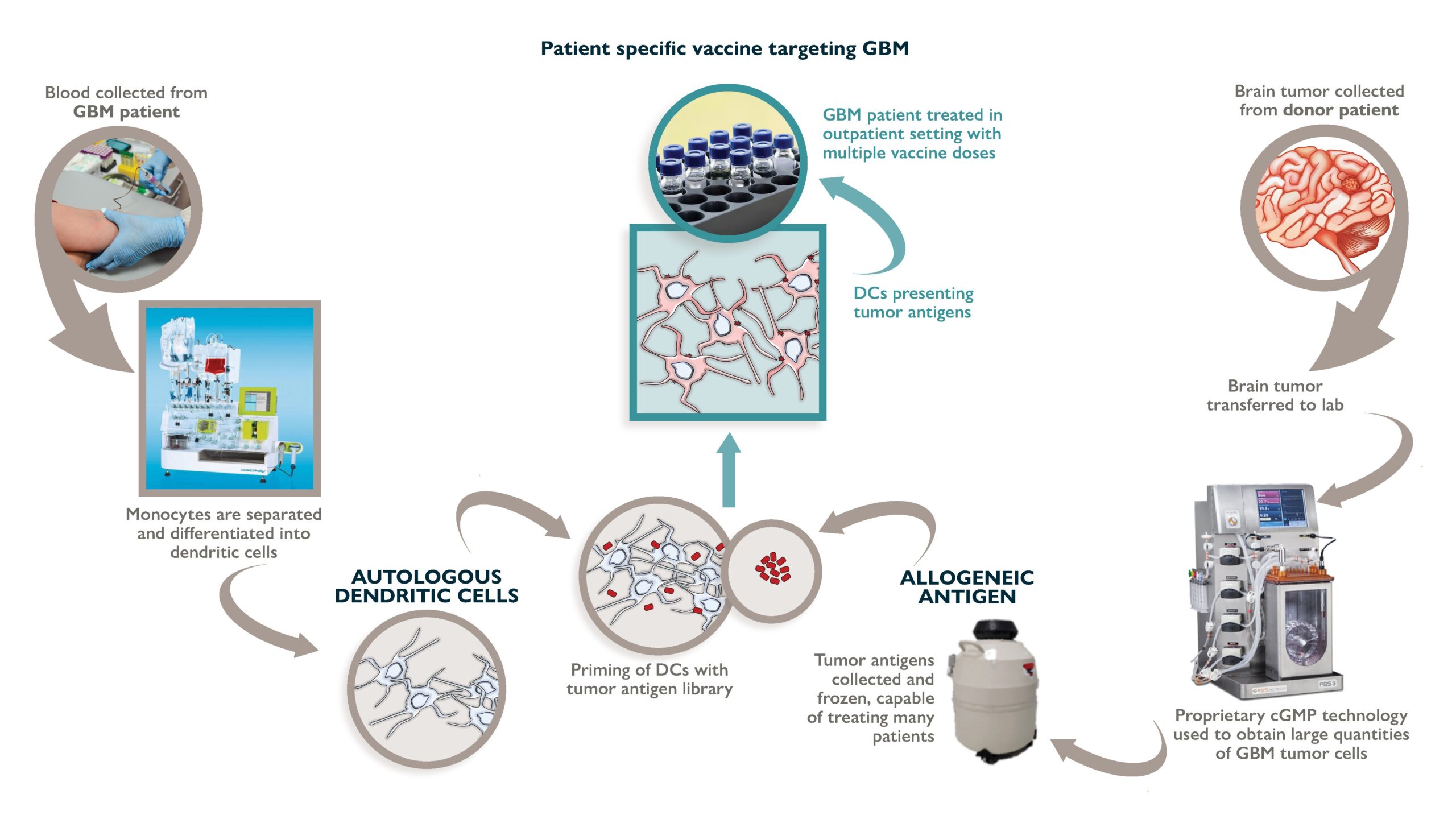
Isolated DCs can be primed with tumor-associated antigens (TAAs) ex vivo (a process often referred to as “antigen pulsing”) to obtain DCs that present highly-expressed, GBM-specific TAAs. Following the pulse, in vitro, DCs can be re-injected into patients as a cellular vaccine to present TAAs to immune effector cells (Figure 1).
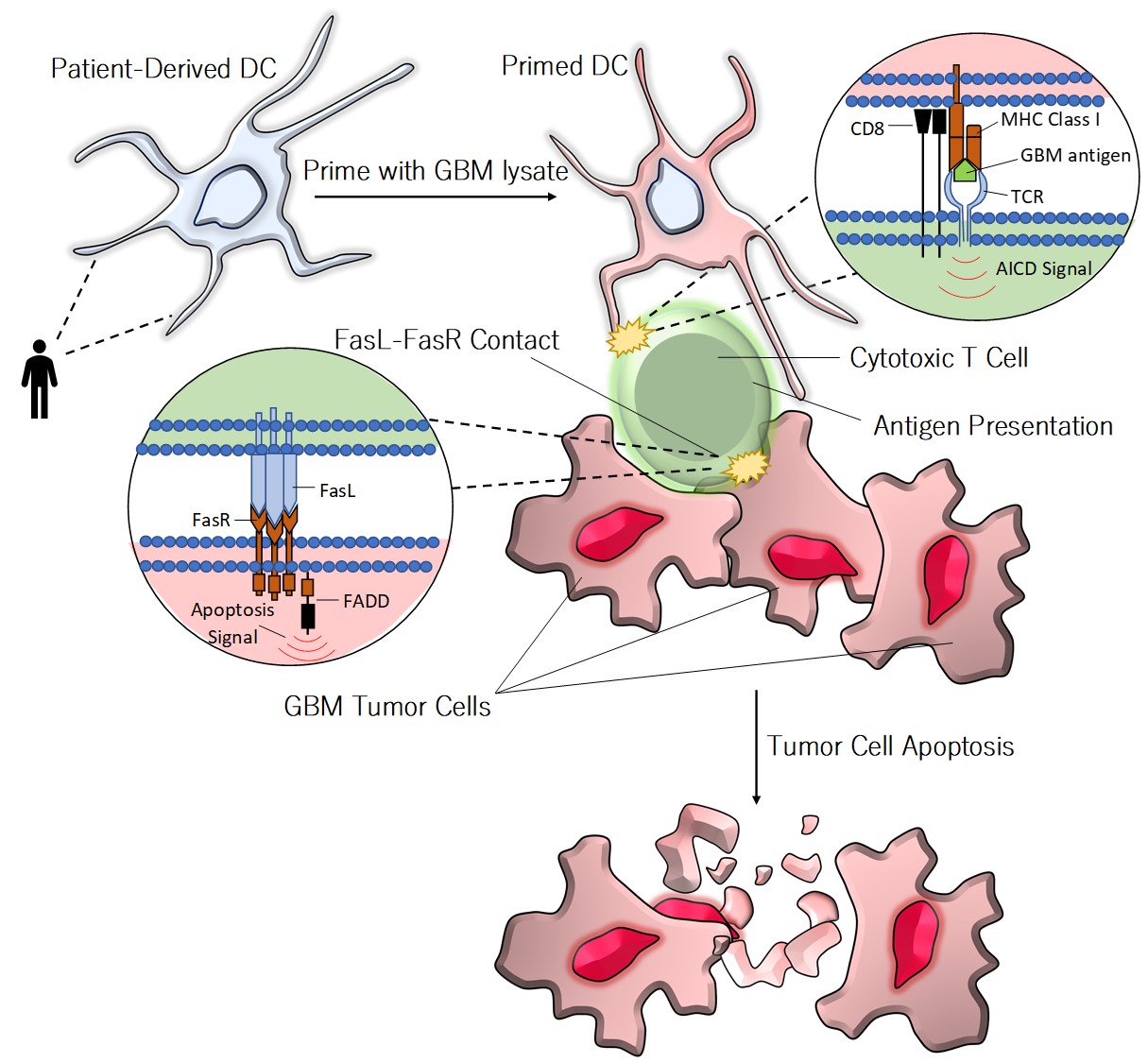
Mechanism of Action
Stimulated T cells will subsequently orchestrate adaptive immune responses against tumor cells featuring the displayed antigens. A diagram depicting the indirect mechanism of DC vaccine mediated tumor cell death is presented in Figure 2.
Injection of DC vaccines supports the generation of anti-tumoral T cells. These vaccine-mediated strategies may have longer-lived oncolytic effects while retaining a higher degree of onco-specificity, thereby limiting toxicity to healthy cells.
DCs present antigens to T cells and facilitate T cell activation. When T cells recognize tumor-associated antigens (TAAs) displayed by a DC, a cascade of molecular events occur, stimulating T cells to differentiate, proliferate, and attack other cells displaying the corresponding TAA.
GBM tumors express a number of antigens that can be recognized by T cells, providing a promising opportunity for cancer immunotherapy.
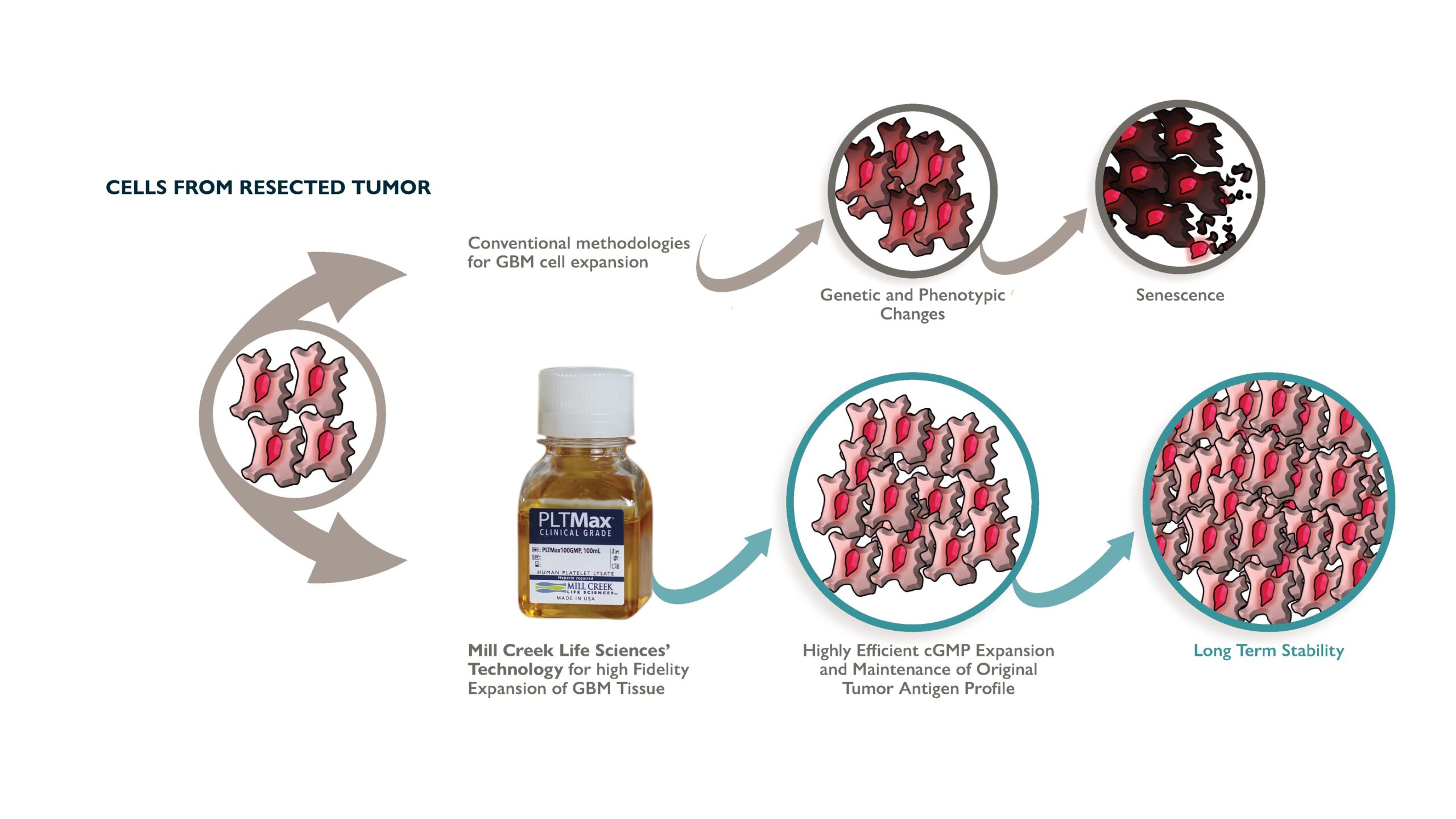
Proprietary and Optimized Cell Expansion
Classical cell culturing methodologies are ineffective for GBM propagation. Traditionally, treatment with autologous vaccines can be delayed several months due to the extensive time required to develop the autologous tumor lysate. Aside from these delays, many patients cannot receive vaccine therapy, due to failure in establishing autologous tumor cell cultures.
The Human Cell Therapy lab at Mayo Clinic established several Good Manufacturing Practice (GMP)-adherent GBM cell lines using PLTMax®, MCLS' proprietary growth supplement derived from human platelets.
PLTMax® maintains a stable doubling time with a minimal genetic drift in expanded cultures (Figure 3), thus maintaining original GBM tumor’s robust and defined tumor antigen expression. This technology allows a highly efficient expansion of GBM primary tissue (as many as 70% of tumors were expandable). Tumor cell lines are the starting material to produce antigen libraries that are used to prime patients’ DC in vitro prior to their re-administration. The high-fidelity expansion of tumor tissue in cGMP compatible format has never been done before and breaks down a significant barrier to tumor immunology.
While standard DC culture techniques produce >90% mature (CD83+) DCs from healthy donor monocytes, <60% mature DCs are obtained from GBM patients' monocytes (P<0.001). However, our proprietary, optimized technique can produce >90% mature DCs from GBM patients using a CliniMACS Prodigy®.
Preliminary data from a phase I clinical trial (MC1272) conducted at the Mayo Clinic (data submitted for publication) shows promising results with a significant increase on progression free survival rate on patients that received the DC Vaccine.
Technology Transfer and Commercial Scale Production
Currently at MCLS, we are evaluating the use of small Vertical-Wheel bioreactors and different cell carriers to optimize tumor cell culture conditions for large scale expansion in suspension (Figure 4). With the optimized protocol, we will establish new GBM tumor cell lines from dozens of patients and characterize them to create a large antigen database.
The use of NextGen Sequencing will allow us to eventually match our preexisting antigen libraries with patients’ tumor profiles for a feasible patient specific, tumor focus therapy. To enhance the immune response, we will explore the combination of the therapy with different adjuvants and immune stimulators.
